Is Splenda really Splendid?
 Splenda, that wonderful trick on nature that allows us to have our cake and eat it too. Unlike its predecessor Aspartame (NutraSweet), it can hold up to cooking temperatures and not breakdown – It can probably hold up to a nuclear blast as I think nothing can break this crap down! People claim it tastes just like sugar, but I think it taste like a sugar and aspirin combination. I accidentally drank some in a beverage once and gagged and tossed the rest of the drink away. But for those who like a little pharmaceutical taste with their confections or just love the taste of sweets so much they can tolerate the bitter after taste – Splenda seems like a real cheat on nature. But is Splenda really that splendid in the larger picture? Let’s take a look at what we know, and more importantly what we don’t know yet.
Splenda, that wonderful trick on nature that allows us to have our cake and eat it too. Unlike its predecessor Aspartame (NutraSweet), it can hold up to cooking temperatures and not breakdown – It can probably hold up to a nuclear blast as I think nothing can break this crap down! People claim it tastes just like sugar, but I think it taste like a sugar and aspirin combination. I accidentally drank some in a beverage once and gagged and tossed the rest of the drink away. But for those who like a little pharmaceutical taste with their confections or just love the taste of sweets so much they can tolerate the bitter after taste – Splenda seems like a real cheat on nature. But is Splenda really that splendid in the larger picture? Let’s take a look at what we know, and more importantly what we don’t know yet.
Splenda contains a man-made compound named sucralose. Sucralose is about 600 times sweeter than sugar. The amount needed to sweeten your coffee would be so tiny, that you wouldn’t be able to get it out of the little yellow packet because static would bind the dust to the side of the paper. So to solve this problem, the manufacturer adds filler in the form of dextrose, sucrose or maltodextrin, which are sugars, giving each pack about four calories – even though they claim zero calories. The manufacturer claims that Splenda taste like sugar, because it’s made from sugar. So how much processing does sugar go through to become sucralose? The following is the recipe for making sucralose. Try to make it at home:
- Sucrose is tritylated with trityl chloride in the presence of dimethylformamide and 4-methylmorpholine, and the tritylated sucrose is then acetylated with acetic anhydride.
- The resulting sucrose molecule TRISPA is chlorinated with hydrogen chlorine in the presence of toluene.
- The resulting 4-PAS is heated in the presence of methyl isobutyl ketone and acetic acid.
- The resulting 6-PAS is chlorinated with thionyl chloride in the presence of toluene and benzyltriethylammonium chloride.
- The resulting TOSPA is treated with methanol in the presence of sodium methoxide to produce sucralose.
Ahhhh… just the way grandma used to make it. Hardly the idea that is suggested when the package states; “Tastes like sugar because it’s made from sugar.”. Being made from sugar gives the impression of something that’s natural. This is nothing nature would have the audacity to create, because it serves no purpose. I am confused as to why anyone would consume mass quantities of a substance that has no nutritional value and is not even a food by any definition of the word.
Sucralose is a sugar molecule that does not exist in nature. Sucralose begins its journey as a sucrose disaccharide (meaning it’s made of two simple sugars or monosaccharides). The two sugars in sucrose are glucose and fructose. Sucrose is the sugar found in fruits, honey, cane, beets and syrups, including HFCS. Through an elaborate chemical process that would make any mad scientist proud, the stereochemistry of the glucose molecule is changed, making it more resemble galactose. A fructose/galactose disaccharide is not anything commonly found in food, so how is the body to deal with such a monstrosity? The real secret to sucralose is that the final product replaces the three oxygen and hydrogen atoms at the end of the now deformed glucose molecule with chlorine molecules, making the compound a organochlorine.
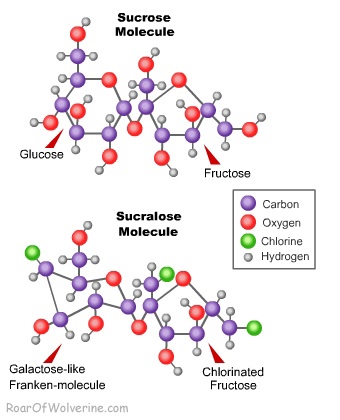 Organochlorines have historically had a bad reputation. Usually only used as a pesticide, they would include a family tree containing chlordane, DDT, Agent Orange and PCBs. All of these compounds were such a disaster, they have been banned from usage. Sucralose was invented accidentally while trying to create a new pesticide. The worse attribute of organochlorines is their resistance to biodegradation, causing an accumulation of the compound in the environment. Supporters of Splenda’s safety will argue that the chlorine (a compound toxic to all living things) is of no threat to the consumer, because the human body can’t break down sucralose and release the chlorine into the tissues. I am not going to follow along with the typical scare tactic of the chlorine causing health problems. After all, the body cannot metabolize the sucralose, so the chlorine never reaches the cells. Although, the FDA final report on sucralose states that 11 to 27% is absorbed by the human body and has a half-life in the blood of 3–5 hours. The Japanese Food Sanitation Council found that the body can metabolize up to 40% of sucralose, which if true, could be a health risk to those who consume a lot of it. [link] But until more information and studies are released on this, I will not use this argument.
Organochlorines have historically had a bad reputation. Usually only used as a pesticide, they would include a family tree containing chlordane, DDT, Agent Orange and PCBs. All of these compounds were such a disaster, they have been banned from usage. Sucralose was invented accidentally while trying to create a new pesticide. The worse attribute of organochlorines is their resistance to biodegradation, causing an accumulation of the compound in the environment. Supporters of Splenda’s safety will argue that the chlorine (a compound toxic to all living things) is of no threat to the consumer, because the human body can’t break down sucralose and release the chlorine into the tissues. I am not going to follow along with the typical scare tactic of the chlorine causing health problems. After all, the body cannot metabolize the sucralose, so the chlorine never reaches the cells. Although, the FDA final report on sucralose states that 11 to 27% is absorbed by the human body and has a half-life in the blood of 3–5 hours. The Japanese Food Sanitation Council found that the body can metabolize up to 40% of sucralose, which if true, could be a health risk to those who consume a lot of it. [link] But until more information and studies are released on this, I will not use this argument.
The real problem with sucralose is the mechanism that makes it work as a sugar substitute – the fact that nothing living can break it down. Studies done on rats have shown that the rodents fed sucralose had a 50% reduction in gut bacteria. [link] This could be something to consider. No human studies have yet been conducted, but I cannot see why human gut bacteria (which are mostly the same bacteria found in rat colons) would fare any better against this substance. So anyone eating yogurt sweetened with Splenda in hopes of restoring gut flora are kind of like a dog chasing its own tail.
Whenever anything we eat is not digested or absorbed, the bacteria within the colon will attempt to feed on it. Oligosaccharides (fiber) are also indigestible. When these natural carbohydrates reach the colon undigested, the bacteria begin to ferment and convert them to butyric acid, a short chain fatty acid used by the cells of the colon. But, when sucralose reaches the large intestines undigested, the bacteria can’t deal with it in any way. The rat study would suggest that the bacteria may die-off in the attempt to metabolize it. So what happens next is that the sucralose passes out with the stool, unchanged. The percentage of sucralose that is absorbed into the bloodstream, is filtered out by the kidneys and passes with the urine. If you eat sucralose, then you are defecating and urinating sucralose with each trip to the bathroom. You’re probably saying to yourself; “So, I have sweet tasting urine and poop and what’s wrong with that?”.
Studies have proven that modern waste treatment does not remove the sucralose from waste water. Details on the study here. So this sweet frankenfood is finding its way back into the water supply. Sucralose breaks down very slowly, if at all, in nature and we have absolutely no idea of its impact on the environment yet. I would imagine that in time, our water will begin to have a sweet (and aspirin) flavor. Look, if someone insists on being the subject of a giant experiment by the food manufacturers and risk possible side effects because they can’t tame their sweet tooth, then fine. But what about those of us who choose not be a corporate guinea pig and are suspicious of the safety claims of sucralose. They’re telling us and every other animal on the planet, that they don’t give a damn and we will have to learn to enjoy their second-hand franken-sweets and share in whatever health risks that they’re willing to take to satisfy their never-ending lust for sweets.
Everyone bitches about second-hand smoke, but no one is contemplating the effects of second-hand sucralose. What if the bacteria in the rat colons are an indication of what could happen to the bacteria in the top soil if sucralose builds up over time from irrigation? How will crops be affected by high concentrations of sucralose in their water? These are serious questions that no one has the answers to at this time, and unfortunately, no one seems to care. Do we have to spend billions of dollars inventing and implementing waste water modifications just so some people can have an artificial sweetener? Like I said at the beginning of this rant, the things we don’t know about sucralose may be the most alarming. If someone can’t apply moderation when it comes to sweets, they should at least eat sugar, aspartame or better yet, stevia. These can at least break down quickly and stop at the end-user. Even though excessive sugar consumption can cause obesity, diabetes and heart disease, at least they won’t be pissing their indestructible organochlorines all over the rest of us who can practice self-control. Then they alone are the one gambling a health risk, not the entire planet.